Material Testing Insights
Read articles by our experts and guest authors on the practical implications of regulations, scientific advancements, and the ideal uses of different analysis methods.
Top articles

Biocompatibility testing of medical devices according to ISO 10993
Some biocompatibility testing according to ISO 10993 is typically needed to bring new medical devices to market in the EU and the US.

PFAS regulations in the EU: overview of restrictions and compliance testing options
The use of toxic PFAS compounds has been regulated in the EU for over a decade, with restrictions getting stricter each year.

EN 13501-1 standard for fire classification of construction materials: Overview of performance classes & criteria
In most cases, several reaction-to-fire tests are required to determine the correct fire performance class for construction products according to EN 13501-1.
Testing to meet EU Monitoring and Reporting Regulation (MRR) requirements
Laboratory analyses required to comply with the MRR often include biomass fraction, net calorific value, and carbon content measurements.
Keep reading >
Standard methods for the electrical testing of plastics and polymers
Ensuring the safety and performance of plastics in electrical applications requires rigorous testing based on ASTM, IEC, and EN standards.
Keep reading >
Lola&Lykke partners with Measurlabs to ensure breast pumps are safe for babies
Breast pump safety testing must account for structural complexity, a vulnerable user group, and stringent regulatory requirements.
Keep reading >
PFAS testing methods and standards: How to select the most appropriate analytical approach?
We summarize current best practices for PFAS methods selection, including available analytical techniques and standard methods for selected sample matrices.
Keep reading >
Overview of UN/ECE Regulation No 118 for fire testing of bus interior components
One or more tests described in Annexes 6–10 to UN/ECE R118 are generally required for materials to be used in bus interior, heating, or engine compartments.
Keep reading >
Overview of ISO 11607 standards for medical device packaging validation
ISO 11607-1 and ISO 11607-2 lay down internationally recognized requirements for the packaging systems of terminally sterilized medical devices.
Keep reading >
Okmetic’s long-standing collaboration with Measurlabs drives smoother R&D projects
Okmetic has turned to Measurlabs for over five years to purchase advanced surface characterization services to supplement in-house test results.
Keep reading >
Nitrosamine impurity analysis of pharmaceuticals by EMA and FDA guidelines
Pharmaceutical manufacturers must ensure that their products do not contain nitrosamine impurities in amounts exceeding the limits set by the EMA or the FDA.
Keep reading >
Cleaning validation of reusable medical devices: how to fulfill EU and FDA requirements?
Rigorous cleaning validation - conducted according to international standards - is crucial to ensure safety and obtain regulatory approval for reusable devices.
Keep reading >
New Construction Products Regulation: overview of requirements and compliance testing
Product-specific testing requirements for construction products will be gradually updated to match the new CPR when new technical specifications are prepared.
Keep reading >
Medical device extractables and leachables testing by MDR requirements
Characterization of extractables and leachables helps medical device manufacturers evaluate the risks that arise from the chemical composition of the device.
Keep reading >
PFAS in food packaging: compliance testing by EU and US regulations
PFAS compounds are being phased out of food packaging on both sides of the Atlantic with regulations such as the new EU PPWR and California Code § 109000.
Keep reading >
Metsä Spring teamed up with Measurlabs to ensure the compliance of fiber-based Muoto packaging
Measurlabs' expertise played a key role in ensuring that Metsä Spring's novel packaging solution fulfills European food contact compliance criteria.
Keep reading >
PFAS-free testing for consumer products: how to verify the absence of forever chemicals
As there are thousands of chemicals within the group, substantiating PFAS-free claims requires both targeted analysis and fluorine content testing.
Keep reading >
Trace elemental analysis of thin films and silicon wafers: A method comparison
Tiny contaminant concentrations can compromise the functionality of semiconductor components, making trace elemental analysis crucial for quality control.
Keep reading >
Viscosity testing techniques, standards, and applications
Choosing the most appropriate viscosity testing technique, based primarily on sample properties, is key to obtaining accurate and reproducible results.
Keep reading >
EN 13501-1 standard for fire classification of construction materials: Overview of performance classes & criteria
In most cases, several reaction-to-fire tests are required to determine the correct fire performance class for construction products according to EN 13501-1.
Keep reading >
SEM vs TEM: How to choose the most suitable electron microscopy technique for your application
Scanning electron microscopy and transmission electron microscopy are both powerful techniques for high-resolution imaging, but they have some key differences.
Keep reading >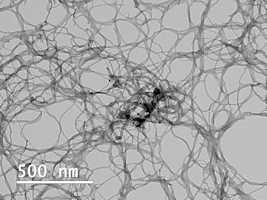
Overview of ISO 16094 standards for microplastic analysis of water samples
ISO 16094 standards will outline the procedures for microplastic analysis of clean water samples using vibrational spectroscopy and thermoanalytical methods.
Keep reading >
Cosmetic packaging testing by EU regulations and industry guidelines
The safety and compliance of cosmetic packaging are frequently assessed using migration tests adapted from those employed for food contact materials.
Keep reading >












