Nanomaterials may only be used in food products in the EU if they have passed a thorough safety assessment by the European Food Safety Authority (EFSA). For EFSA to perform the assessment, a comprehensive set of data on the product must be provided, including information on its physicochemical properties and toxicological profile.
Conventional materials, such as different food powders, may also require testing to rule out the presence of nanoparticles. Measurlabs offers the necessary tests to determine whether substances and mixtures used in food and feed production require a nano-specific risk assessment, as well as further tests to assess the properties of materials that do have nanoscale properties.
Definition of a nanomaterial
The official EU definition of a nanomaterial is outlined in Commission Recommendation 2022/C 229/01 from June 2022.1 A material is classified as a nanomaterial if it consists of solid particles, either on their own or as identifiable constituents in aggregates or agglomerates, where at least 50% or more of the particles fulfill at least one of the following conditions:
At least one external dimension is in the size range of 1–100 nm.
In the case of particles with an elongated shape, two dimensions are smaller than 1 nm and one dimension is larger than 100 nm.
In the case of plate-like particles, one dimension is smaller than 1 nm and the other dimensions are larger than 100 nm.
Particles with at least two dimensions measuring more than 100 μm are not counted as nanoparticles. Also, even if the size criteria are met, materials with a specific surface area by volume of less than 6 m2/cm3 are not considered nanomaterials.
In addition to materials that meet the nanomaterial definition, EFSA also requires a nano-specific risk assessment to be performed for conventional materials that contain a fraction of small particles or have nanoscale properties. The cut-off point for small particles is 250 nm, as there is evidence that the gastrointestinal tract may absorb particles up to this size.2
When does a conventional material require a nano-specific risk assessment?
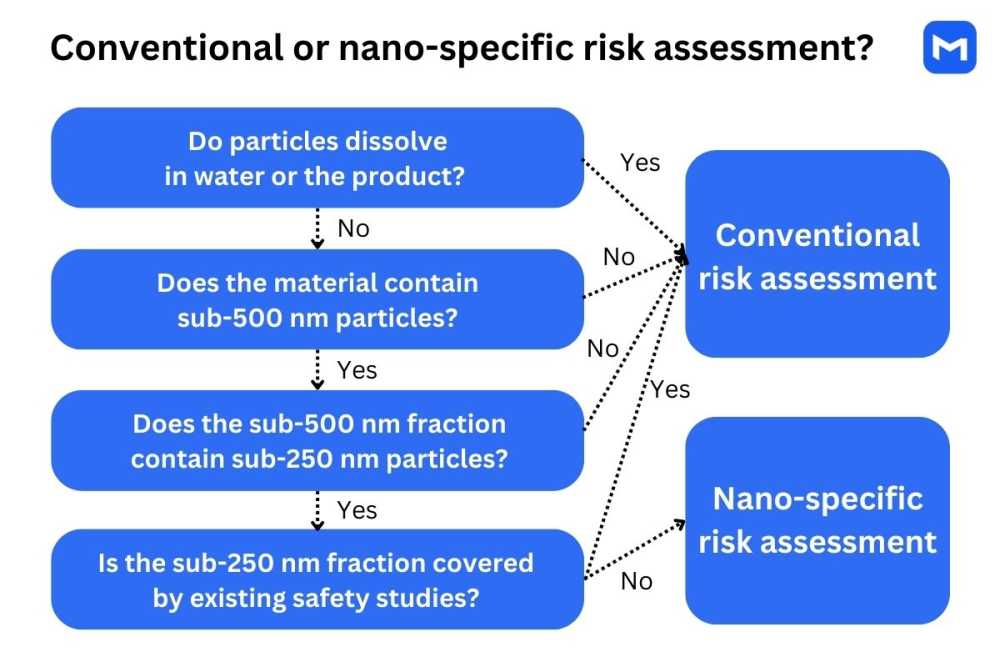
The procedure for determining whether nanoscale properties should be accounted for during the risk assessment is outlined in a 2021 EFSA document known as SC Guidance on Particle-TR. The process consists of three steps:3
Determination of solubility and dissolution rate: If solubility in water is ≥ 33.3 g/L, if ≥80% of particles dissolve in water in 30 mins, or if particles are fully dissolved in the marketed product, it can be assumed that consumers are not exposed to nanoparticles. No further analysis relating to nanoscale properties is needed.
Particle size distribution analysis: If the material contains less than 10% of small particles (<500 nm), or if less than 10% of the sub-500-nm particles are smaller than 250 nm, the risk of nanoparticle exposure is low, and no further analysis is needed.
Overview of existing safety studies: If existing studies on a similar material demonstrate that the found fraction of small particles does not pose safety concerns, additional tests are not needed. The existing studies must have been conducted according to EFSA or OECD guidelines on a material that contains a similar fraction of nanoparticles.
The above procedure applies to chemical substances and mixtures used in food, feed, and supplement production. In the case of multi-constituent mixtures, each component needs to be evaluated individually in addition to the full material.
Particle size distribution analysis by EFSA guidelines
A particle size analysis is only needed if the solubility and dissolution rate criteria described above are not met. The analysis is generally performed using scanning electron microscopy (SEM), although other applicable methods, such as centrifugal liquid sedimentation, nanoparticle tracking analysis, or filtration may be used for the initial screening of sub-500 nm particles.
The sample preparation procedure needs to be documented in detail to demonstrate that the sample is representative of the material in the form that consists of the smallest possible dispersible particles. This entails following a dispersion protocol that minimizes particle agglomeration and aggregation.
When using SEM, it should be demonstrated that the magnifications are high enough to detect nanoparticles. The analysis should provide the following information:
A rough estimate of the particle size distribution
Agglomeration and aggregation status
General morphology
Surface topology
Structure (crystalline vs. amorphous)
Information on possible contaminants
If the sample contains sub-500 nm particles, EFSA recommends always performing a quantitative analysis with electron microscopy, if technically feasible. If a small fraction (< 10%) of sub-250 nm particles is found, it needs to be shown that their presence is a result of the manufacturing process and that their properties are essentially similar to those of the larger particles apart from size.
Extended risk assessment for nanomaterials in food
If the material is found to contain small particles and existing safety studies do not sufficiently address the risks, a risk assessment by another EFSA document, known as SC Guidance on Nano-RA4, is required. The document outlines the additional testing requirements for addressing nanoscale properties and the related safety hazards. These include:
Detailed physicochemical characterization, including information on the chemical identity and purity, particle size and shape, structure, surface area, density, and porosity of the material. Some of the applicable testing methods include BET analysis for determining the specific surface area and He pycnometry for determining porosity.5
Exposure assessment, including an estimate of the dietary intake of nanoparticles and their degradation products.
Characterization of toxicological hazards, starting with in vitro methods and only proceeding to in vivo methods when strictly necessary.
Nanomaterial analysis by EFSA guidelines
Measurlabs offers several analytical methods for nanomaterial characterization and for determining whether or not a material contains nanoparticles or has nanoscale properties. Most often, our customers use the test results to demonstrate to EFSA that their material does not fulfill the definition of a nanomaterial and that a conventional risk assessment is therefore sufficient.
If you wish to have your product or material tested by the EFSA nanomaterial guidelines, do not hesitate to contact us through the form below. One of our experts will get back to you within one business day.
References:
1 See Commission Recommendation of 10 June 2022 on the definition of nanomaterial (2022/C 229/01). When the EFSA guidelines were written in 2021, they mentioned Regulation (EU) 2015/2283 definition of “engineered nanomaterial” and Regulation (EU) 2018/1881 definition of nanomaterial. Now, the Commission strives to implement the new definition in all sectors.
2 EFSA: Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticles (SC Guidance on Particle-TR), the justification for the 250 nm limit is found in Part 3.3.1.3.
3 The appraisal routes are outlined in section 2.2. of SC Guidance on Particle-TR.
4 EFSA: Guidance on risk assessment of nanomaterials to be applied in the food and feed chain: human and animal health (SC Guidance on Nano-RA)
5 The requirements are described in detail in Table 1A of SC Guidance on Nano-RA.

