Non-intentionally added substances (NIAS) are possibly harmful chemicals that end up in food contact materials (FCMs) without being purposefully added during the production process. These substances may migrate into food and pose safety risks to consumers.
To mitigate the risks, NIAS testing should be performed to detect non-intentionally added substances that are transferred from FCMs into food. Together with other analyses, such as specific and overall migration testing, comprehensive NIAS screening helps ensure that materials are safe and compliant with all relevant EU regulations.
How do non-intentionally added substances end up in FCMs?
NIAS are divided into three categories based on how they end up in food contact materials:
Side products are formed during the manufacturing process as a result of chemical reactions between different components of the food contact article. An example would be a coating reacting with the main body of plastic packaging.
Breakdown products end up in the material when its constituents or additives degrade during manufacturing or use. Typically, the vast majority of NIAS in virgin plastic FCMs are degradation products, such as phenol or benzaldehyde compounds from antioxidant breakdown.
Contaminants include impurities in raw materials and environmental pollutants that end up in the product during different stages of the manufacturing process. Contaminant presence is a particular concern in FCMs made of recycled materials. Recycled plastics are likely to contain oligomers and additives, while recycled paper and board often contain mineral oils, bisphenols, and phthalates.
For more technical insights on NIAS testing and risk evaluation, watch our expert webinar.
Regulatory framework for NIAS testing in the EU
According to the Framework Regulation (EC) 1935/2004, food contact materials must not release substances into food in amounts that could endanger human health.1 NIAS testing is one key step in ensuring that this is the case, regardless of material type.
More concrete compliance criteria are outlined in material-specific regulations, such as Regulation (EU) 10/2011 on plastic food contact materials. The regulation requires substances used in plastic FCMs to have a "high degree of purity" and contain “only a minor amount of non-intentionally added substances”.2 This is shown by demonstrating that each identified NIAS either complies with the specific migration limit (SML) specified in Annex I or passes a risk assessment showing that a safe migration level is not exceeded.
How is NIAS testing performed?
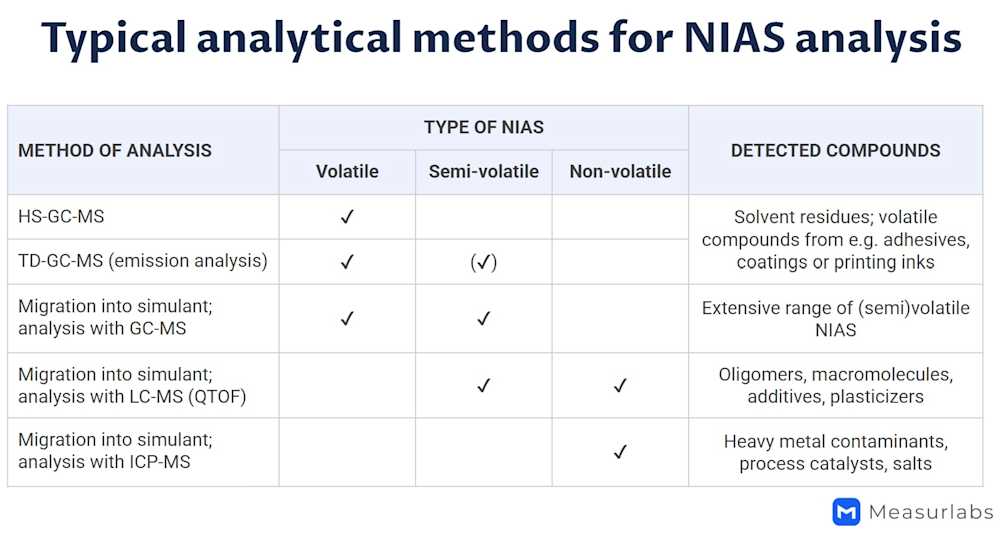
The first step in NIAS assessment is a general screening that identifies non-intentionally added chemicals without prior information on what could be found. This is usually done using GC-MS-based methods, which are effective for detecting a wide range of volatile and semi-volatile organic compounds. If the material is suspected to contain oligomers and other non-volatile organic NIAS not detectable with GC-MS, these should be analyzed with an LC-MS-based method. For inorganic NIAS, ICP-MS is typically used.
The initial NIAS screening is semi-quantitative and typically has a limit of quantification (LOQ) of 0.01 mg/kg (=10 µg/kg). If traces of substances are detected in lower concentrations, they are generally considered safe and do not require further assessment. However, if there is reason to believe that potentially carcinogenic or mutagenic substances with a lower safety threshold may migrate from the material, additional targeted analyses are needed to ensure migration does not exceed safe limits.
How are the results evaluated?
The way that detected NIAS should be dealt with depends on whether they are specified in Annex I to Regulation (EU) No 10/2011. If a substance listed in the annex is discovered during NIAS screening, its migration to a food simulant is assessed similarly to any intentionally added substance and compared with the applicable specific migration limit. As the initial screening is semi-quantitative, this step typically includes a further substance-specific analysis to quantify the level of migration more accurately.
If the detected NIAS is not listed in Annex I to Regulation No 10/2011, its safety should be assessed according to internationally recognized scientific risk assessment principles.3 Examples of references that can be used to determine acceptable migration thresholds include:
National regulations that list a wider range of authorized food contact substances, such as Swiss Ordinance SR 817.023.21 (Annex 10)
Maximum tolerable concentration at the tap (MTCtap) values specified in the Drinking Water Directive (EU) 2020/2184
European Food Safety Authority’s (EFSA) tolerable daily intake (TDI) values
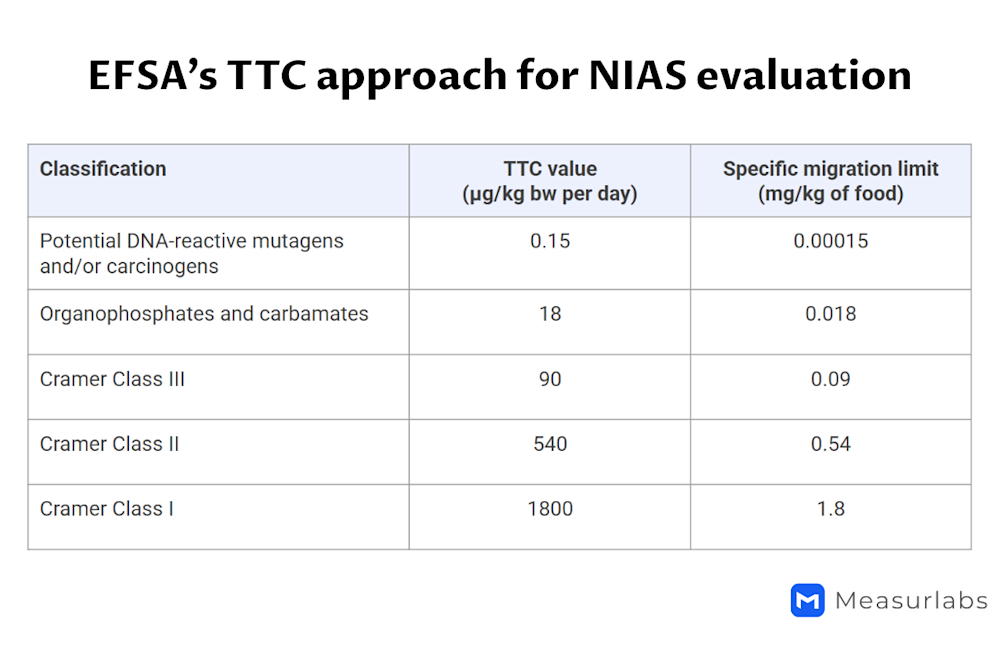
The Cramer classification of substances based on their chemical structure and the threshold of toxicological concern (TTC) approach4
How extensive should the NIAS assessment be?
The extent of the required NIAS assessment depends on material composition, intended use, and the associated risks. As a general rule, most food contact materials should be tested for unintentional heavy metal contamination.5 In addition to this, a general GC-MS screening is often sufficient for low-risk materials, such as common grades of virgin plastics, which tend to pass the screening with none to a few substances of concern identified.
When food contact materials contain recycled plastics or fibers, the efficiency of the cleaning process influences the likelihood of impurities being found. As the risks are higher, NIAS testing should be performed more frequently, and it should include several analyses, such as LC-MS screening for non-volatile NIAS and targeted analyses for specific contaminants.
Table 1: Selected NIAS that require targeted analysis
Substance/substance group | Examples of applicable materials |
Primary aromatic amines (PAA) | Substances that contain aromatic isocyanate groups, polyurethane-based materials, azo dyes, and recycled plastics |
Phthalates | Recycled paper and board |
Bisphenols | Recycled plastic, paper, and board |
Mineral oils (MOSH and MOAH) | Recycled paper and board |
Residual volatile organic compounds (VOCs) | Printed packaging, recycled paper and board |
N-nitrosamines and N-nitrosable substances | Rubber and elastomers |
Our NIAS testing solutions
Measurlabs offers a comprehensive range of NIAS testing options for companies working with various types of food contact materials. Popular services include:
Several heavy metal analyses for different material types, including specific migration of elements listed in Annex II to Regulation (EU) 10/2011, metal release from paper and board according to BfR Recommendation XXXVI, and migration of heavy metals from ceramic, glass, and enamel according to Directive 84/500/EEC and several national regulations
Low-detection limit analyses for bisphenols A, B, F, S, and AF, toluene residues, and phthalates & plasticizers
Extended VOC screening to evaluate the decontamination efficiency of recycling processes
If you require a NIAS assessment or any other food contact material testing services, do not hesitate to contact our experts using the form below. We reply to queries within one business day.
Notes and references:
1 Framework Regulation (EC) No 1935/2004, Article 3
2 The requirement for a high degree of purity is laid down in Article 3a of Regulation (EU) No 10/2011.
3 This requirement is laid out in Regulation (EU) No 10/2011, Article 19.
4 In the Cramer classification system, substances are divided into three classes according to the toxicological concern they are likely to pose based on their structure. The migration limits are as follows:
1.8 mg/kg for Class I
0.54 mg/kg for Class II
0.09 mg/kg for Class III
In addition, a limit of 0.018 mg/kg applies to organophosphates and carbamates, and a limit of 0.00015 mg/kg to potentially mutagenic or carcinogenic substances.
5 In addition to Annex II of Regulation (EU) 10/2011, strict heavy metal release limits are specified in a range of national regulations used as safety references when harmonized legislation is not available. Examples include BfR Recommendation XXXVI (paper and board) and French Information note n°2012-93 (wood).

